I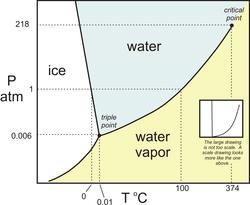 ce is the solid phase of H2O. The phase of pure water depends on the ambient temperature and pressure. At sea level, the atmospheric pressure is 1 atm and ice forms at 0ºC. As pressure increases, the temperature required to freeze water becomes colder, while the temperature required to evaporate water rises.
ce is the solid phase of H2O. The phase of pure water depends on the ambient temperature and pressure. At sea level, the atmospheric pressure is 1 atm and ice forms at 0ºC. As pressure increases, the temperature required to freeze water becomes colder, while the temperature required to evaporate water rises.
If different solutes mix with the water, then the temperature at which the solution freezes declines. On average, the ocean contains 35 ppt dissolved salt. The salinity of the ocean lowers the temperature at which water freezes.
Created by Hanna Asefaw on Monday, 12 December 2016 and updated on Saturday, 17 December 2016
- 1098 reads