FeMO2 Dive Cruise 2007
Report -- In Situ Electrochemistry at Loihi and the AIS ISEA™ III
FeMO2 Dive Cruise 2007 |
The purpose of the ISEA (In-Situ Electrochemical Analyzer) is to be able to describe chemically the environmental habitat of micro and mega fauna at the bottom of the ocean. The instrument can also be used to investigate diffuse and hydrothermal vent sites to aid geochemists in characterizing these very different underwater environments. After several SBIR grants we have perfected the AIS ISEA™ III, this instrument is truly a state analyzer capable of analyzing data from voltammetric sensors, potentiometric sensors, CTD instruments, temperature, pH, and so on. The instrument has the ability to multiplex up to four analog signals simultaneously, in combination with several auxiliary channels allowing for very flexible data acquisition.

The basis of the instrument is electrochemistry. We look at the oxidation and the reduction of metal and complex species in solution. The type of electrochemistry that is performed by the ISEA is called voltammetry. Electrochemistry requires a supporting electrolyte, that is, it requires something to conduct electrical current. Seawater is an excellent electrolyte and various chemical species can be determined in-situ down to nano-molar levels.

The instrument works by applying a potential between a working electrode and reference electrode, whereby currents are measured between both electrodes. Different elements (ions) generate different currents at different potentials (voltages). As we scan the potential at the working electrode, various electrochemically active species (elements, ions) in the solution under study (seawater) can oxidize (lose electrons) or reduce (gain electrons).
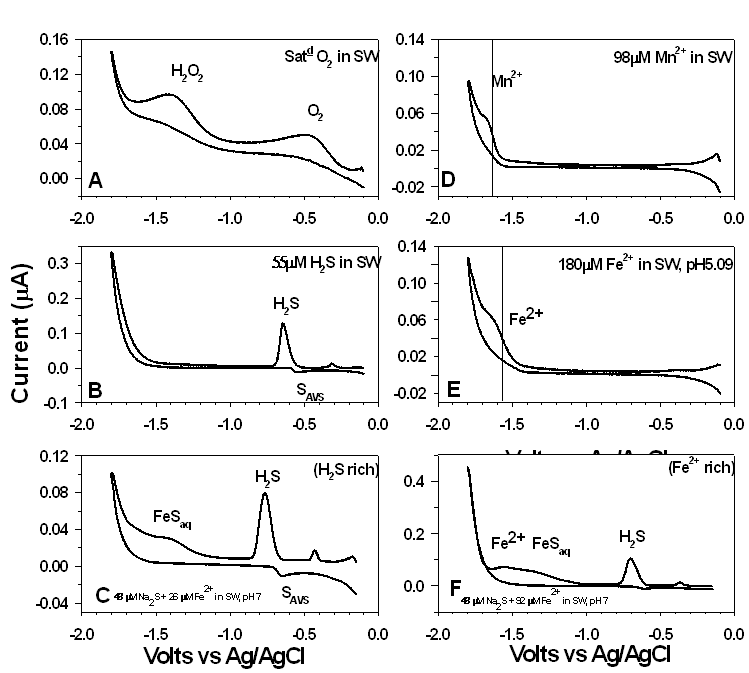
These redox reactions are recorded by the ISEA as small currents. This is analogous to spectroscopy where the wavelength is scanned and the absorbance of a chemical species is recorded. In both techniques the position of the peak is indicative of what the substance is and the height of the peak tells you the concentration or abundance. By running standards we know what chemical species are undergoing oxidation or reduction, and the standards also allow us to quantify the amount of chemical species under study.
Although electrochemistry seems simple, the implementation of it to deep-sea research has been a challenge. The miniaturization of the instrumentation, the large amount of data collected and the electrodes used are constantly being updated and revised for more extreme tasks at the bottom of the ocean. So far we have successfully deployed our voltammetry to 5,000 meters and plan on taking these systems to the deepest part of the ocean, in the Marianas Trench, sometime in the future.

I wish to acknowledge my colleagues whose help has been appreciated over the years during my instrument development: Dr. George Luther, Dr. Brian Glazer, Dr. Martial Tallifert and Dr. Greg Druschel. If you want to read more about our instruments, please visit http://aishome.com.
Don Nuzzio onboard the R/V Kilo Moana
Below you can see the typical voltammograms we record in-situ in the oceans and at hydrothermal features.
| FeMO2 Cruise Home Page |