FeMO3 Dive Cruise 2008
Report -- Colonization of Microbial Mats by Different Types of Chemosynthetic Bacteria
FeMO3 Dive Cruise 2008 |
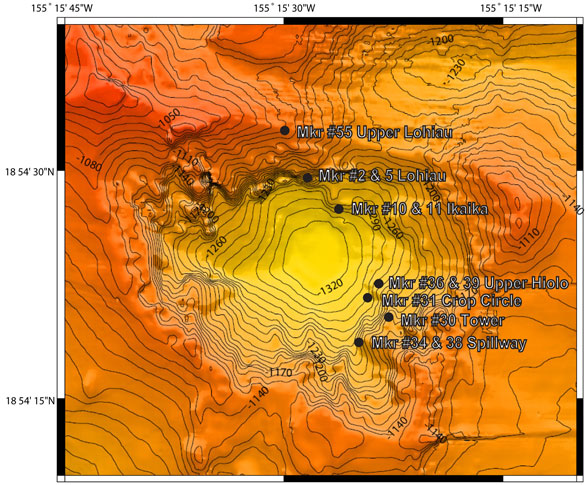
My laboratory studies the microbial ecology of hydrothermal vent systems and we are very interested in the changes that occur within these microbial communities over time and space depending upon environmental conditions. This is much like taking the microbiological census as well as figuring out what everyone likes to order off the metabolic menu. Over the past few years we have seen a dramatic transition in and around Pele’s Pit regarding the microbial mats that form at hydrothermal vents (see Pele’s Pit Map).
We have been able to track this overall shift in microbial community structure using a DNA fingerprinting technique called terminal-restriction fragment length polymorphism or T-RFLP for short. This molecular biological tool allows us to target the small subunit ribosomal genes of bacteria, which have been shown to be an excellent genetic benchmark determining their relatedness. In a nutshell, it allows us to tell the difference among the butterflies and the elephants in terms of microorganisms, which often look very similar under the microscope.
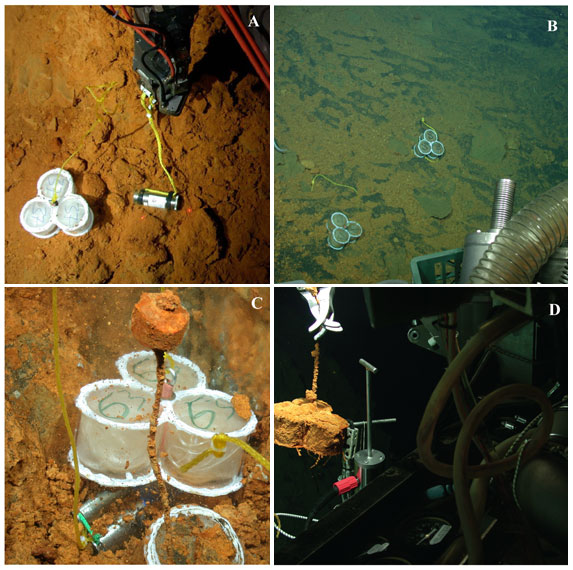
One of the primary environmental parameters that we have witnessed is the generalized cooling trend of hydrothermal vent effluent across all the Pele’s Pit Vents. Just after the last eruption in 1996, the northern pit vents at Ikaika had a temperature of up to 200°C. Presently, our hottest temperatures are found in the southeastern pit at the Hiolo Vents area, which has recently been measured at nearly 50°C. In a effort to monitor the active bacteria that are able to colonize the surfaces around the various vents, we have constructed microbial growth chambers which contain ~33 square meters of surface area available for colonizers to grow (see photographs taken with the Jason2 below). We have deployed and recovered these growth chambers and used our DNA fingerprinting to detect what types of bacterial comminutes are able to take advantage of this newly available surface area for growth, where they derive all their nutrients and energy from vent fluids.
What we have discovered is that all the colonizing bacteria are able to gain their energy from inorganic compounds making them what we call chemotrophs. As these compounds are derived from geologic sources (also known as rocks) we also call them lithotrophs AND because they are able to make their own organic carbon compounds from carbon dioxide we also call them autotrophs. Since chemo-litho-autotrophs is a pretty long name, so we shorten it to chemosynthetic. So, even though the bacterial populations that we have been monitoring all along have all been primarily chemosynthetic, we have seen a transition from mostly sulfur-oxidizing communities to ones dominated entirely by iron-oxidizers.
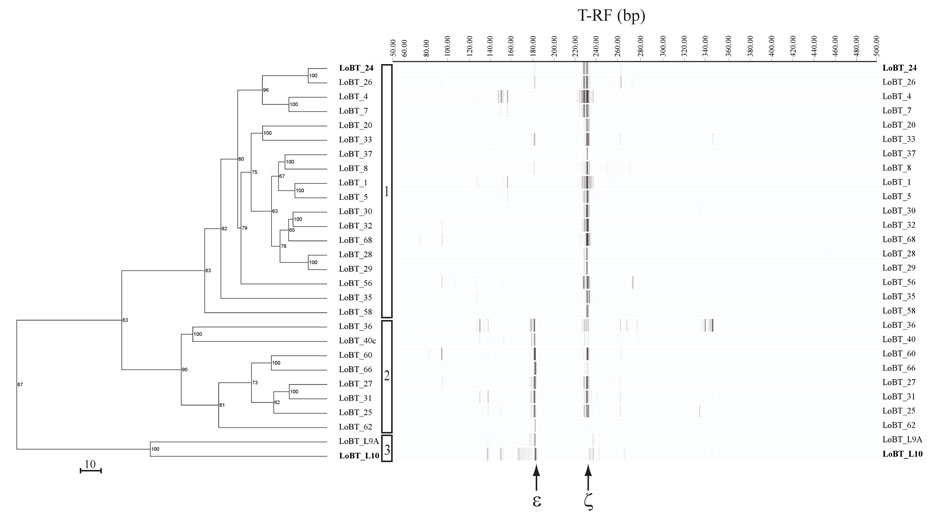
This relates to the environment conditions where after the last eruption, Pele’s Pit Vents were hotter and had more reduced sulfur compounds available in vent fluids, whereas now we measure cooler temperatures along with mostly iron dissolved in vent fluids. An example of this can be seen with our DNA fingerprinting (T-RFLP) data from our microbial growth chambers (see cluster analysis figure on the bottom of this page). Early after the last eruption, we detected more Sulfurimonas (a genus in the class, epsilon-Proteobacteria), which are known for their ability to oxidize sulfur compounds.
We also detected some Mariprofundus (another genus in the class, zeta-Proteobacteria), which are now known to be iron-oxidizers along with the sulfur-oxidizing bacteria. More recently, we have detected an almost exclusive presence of the iron-oxidizing Mariprofundus, which seems to currently dominate at all the vents studied. This was the largest group identified through a process we used known as cluster analysis. We are still trying to tell the different types of iron-oxidizers apart and are working on new and better molecular tools with that goal in mind.
In addition, we did detect a third cluster of community types from only two of our growth chambers. These were deployed just after the eruption when the temperatures were ~100°C. These communities were again enriched with an epsilon-Proteobacteria, however, this time it was dominated by Nitratiruptor, as determined through phylogenetic analysis.
This recently discovered genus is capable of the unusual metabolism of nitrate-reduction through hydrogen-oxidation in the presence of elemental sulfur. Nitratiruptor was first discovered at the Iheya North hydrothermal field in the Mid-Okinawa Trough by Nakagawa and colleagues. We are currently attempting to culture this bacterium from Pele’s Pit so that we might be able to confirm the occurrence of this unusual chemosynthetic metabolism.
| FeMO3 Cruise Home Page |